Global Professionals
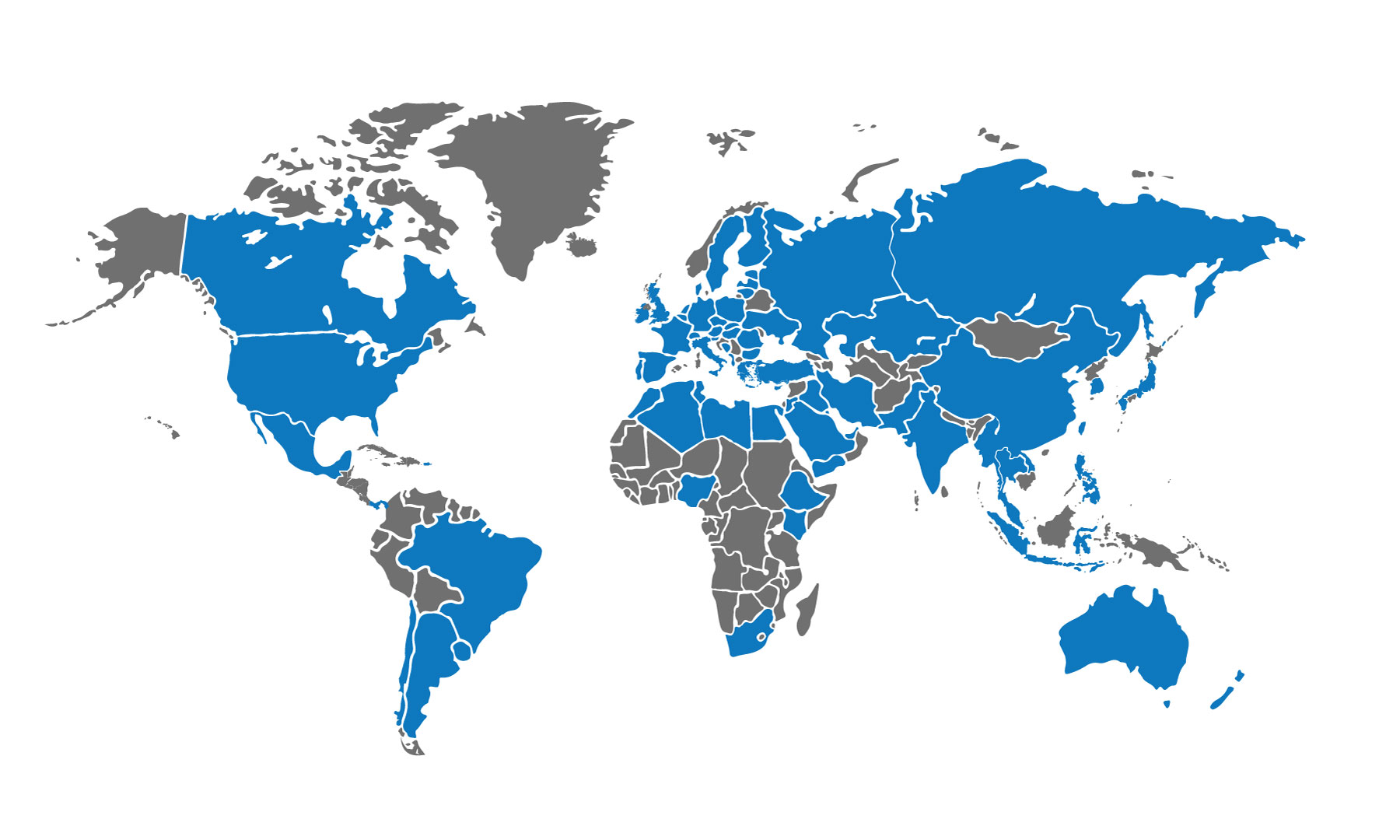
EXPERtS AROUND THE WORLD
We deliver our services by leveraging our global network of proven quality, manufacturing, and regulatory experts in 90+ countries. Our team has the quality skills and commodity expertise to put you on-site faster, eliminate travel budgets, and work in a project-specific capacity.

Walter Stephan
- Aerospace/Defense

Walter brings a wealth of experience from the aerospace/defense industry, having led FACC, a global leader in aerospace composites, as the CEO for 27 years. Walter’s proven track record of driving innovation, improving operational efficiency, and delivering exceptional client service through rigorous quality assurance will be invaluable as we continue to expand our technical services offerings and support our clients in the aerospace, defense, and infrastructure sectors.

Martin Van Trieste
- Life Sciences

An accomplished executive with 40 years of expertise in leading teams dedicated to product development, manufacturing, quality, and distribution of sterile drugs and medical devices. Known for global experience and in-depth knowledge of manufacturing, quality systems, Good Manufacturing Practices, and regulatory standards worldwide, he is a proactive and pragmatic leader skilled at anticipating, troubleshooting, and resolving quality and regulatory challenges. He cultivates a patient-focused, science- and risk-based quality culture, driving high-quality processes and outcomes. His collaborative approach with lawmakers, policymakers, and regulators consistently advocates for patients’ best interests.
As the founder and inaugural CEO of Civica Rx, a U.S. nonprofit pharmaceutical company, he was pivotal in creating its innovative business model focusing on solving drug shortages. He also founded Rx-360, a global pharmaceutical supply chain consortium bringing together biopharmaceutical companies, suppliers, service providers, and regulators.
Recognized as an industry influencer, he was recently named by Modern Healthcare as one of the Top 100 Most Influential People in Healthcare. Medicine Maker honored him as a “Champion of Change” on its Power List for his leadership in Civica. He is a mentor, motivator, and problem-solver specializing in fostering a Culture of Quality.
He currently serves on several boards, including Redica Systems and SmartSkin Technologies, and has previously served on boards such as Civica Rx, Phlow, and INCOG BioPharma Services. As an advisor to executives and their teams, he has helped transform organization and build stronger teams. He has documented his knowledge and experience in his recent book, “Protecting Patients at All Costs: The Drug Watch Dogs.”
Passionate about his mission to prioritize patients’ best interests, he continues to be an advocate and leader in quality and patient safety.

Jeff Luckey
- Aerospace/Defense

Jeff Luckey retired from The Boeing Company in January 2017 after 30 years of service; he now operates JGL Consulting LLC., an aerospace consulting company.
Jeff’s career began to take form in 1987 when he was a member of the McDonnell-Douglas team, where he served as the Supplier Management Director of 717 activities in Long Beach, CA.After McDonnell-Douglas merged with Boeing, he navigated many positions within the supply chain system, most notably leading the supply teams on the 747-8 intercontinental freighter and serving as Vice President of the 787. On the 787 program, Jeff was responsible for the formation of the global supplier team and execution of supplier activities on both the 787 Dreamliner and DreamLifter operations, managing supply chain risks and opportunities, and positioning the supply chain for new airplane derivatives.
In his final years with Boeing, Jeff was based in London and led the Supplier Performance Field Operations teams in Europe, the Middle East, and India.
Jeff was honored to receive the Aviation Week & Space Technology “Laurels Award” for outstanding achievement in the field of aeronautics and propulsion.
Jeff loves to fly and became a Commuter Airline pilot and flight instructor in his early years. In his later years, he enjoys time with his family. He loves being a grandpa, and skiing and snowmobiling.

Carol Ciszek
- Consumer Products

I am a Quality professional with over 35 years in the food and personal care products industries. I joined SQA as a Sr. Technical Advisor in September 2022. Having had many roles across multiple brands/industries I am a strong believer in the importance of Quality across all the many functions and levels of an organization. I am an advocate of building/evolving the Quality organization as a proactive, value-added business partner while maintaining and protecting the company’s core values of product safety and quality. In addition to my Quality responsibilities, I have led/participated in Crisis Management at both Kraft and Estee Lauder and served on the industry’s Personal Care Products Council board for more than 5 years. I am passionate about inspiring/mentoring individuals to achieve their full potential, with outstanding results, and am enjoying helping SQA achieve their goals with thoughtful strategic and customer insights.
Qualified, Specialized, Local

Garrett R.
- Florida

As a former SQA client of 10 years, I decided to become an associate in 2016. At that time, becoming an SQA Associate was the next logical career progression for me. Using my supplier quality management background, spanning 33 years in Aviation, Space, Defense, Medical Device and Pharma, SQA enabled me to contribute to SQA’s equally diverse client base, fulfilling roles in auditing, Project Management and consulting.
Recently, I have been more actively engaged with the SQA – Pfizer partnership conducting many onsite audits for a wide range of materials and products. Not only does this activity fulfill a necessary function for the SQA – Pfizer partnership, it also stretches me to learn so many different approaches to solving similar supplier issues.
As I head into the twilight of my profession, I could not ask for a better suited role to be performing, or the partnership I have with SQA.

Koichi O.
- Japan

I have worked for many years as a quality assurance engineer and manager for a manufacturer that develops and manufactures space systems and equipment. I am currently assisting a U.S. satellite system manufacturer with inspections of their subcontractors in Japan. In the space industry, the contractor is contracted to perform inspections at key events during subcontractor manufacturing and testing. I live in Japan, and by performing inspections in Japan on behalf of the contractor, I help the contractor reduce costs and maintain quality.

Sigit S.
- Indonesia

An experienced quality management professional with over 15 years of expertise in a leading multinational company, demonstrating strong leadership, analytical, and communication skills.

Amel C.
- Tunisia

A holder of Medical Doctor since 1995, I have been working as QMS Responsible at Baxter for 08 years then I started my own business as consultant and trainer in the field of quality management systems. I’m qualified as third-party auditor with DQS for ISO 9001, ISO 13485 & ISO 22716. Since 2011, I’m working as second party auditor with SQA.

Ilias C.
- Switzerland

With 15 years of experience in the life sciences industry, I specialize in Computerized System Validation (CSV), Project Quality Management (PQM), customer and internal audits, and business and technology consulting. I have deep expertise in the validation of computerized systems within GxP-regulated environments, as well as in risk management, remediation assessments, and optimizing global quality, operations, manufacturing, and IT development processes. Certified as a Quality Auditor (CQA) by the American Society for Quality (ASQ), Certificate #75336, I am skilled in conducting ISO and GxP audits for suppliers to life sciences companies. I have a proven ability to lead cross-functional teams, driving projects to successful completion on time and within budget, leveraging Lean Six Sigma, Agile/Scrum, and Waterfall methodologies. With strong facilitation and coaching skills, I consistently deliver results.

Jim Q.
- China

Jim Qi brings 23 years of experience in quality assurance and regulatory compliance within the medical device and pharmaceutical industries. With a deep expertise in ISO 13485/9001 and FDA 21 CFR 820/210/211, he has conducted numerous audits for healthcare industry clients. His specialties include FDA inspection readiness preparation, remediation of FDA warning letters, 510(k) submissions, product sterilization, and CE Technical Construction File (TCF) compliance. An esteemed member of ASQ, Jim was honored with the 2009 ASQ Outstanding Membership Award. He holds certifications as an FDA QSR/ISO 13485/9001 trainer and is a Lean Six Sigma Black Belt. Outside of his professional work, Jim enjoys reading, writing, traveling, swimming, and playing table tennis.

Renata V.
- Czechia

Auditing is my true passion! I am a Doctor of Pharmacy with 20 years of experience in the medical device and pharmaceutical industries, having held various technical and quality-focused roles. My career began in the analytical laboratory, followed by roles in pharmaceutical development and production, and later expanding into the quality management of Class III medical devices. Currently, I work as an independent auditor, specializing in medical device and pharmaceutical supplier and internal audits. I also serve as an auditor for Certification Bodies and Notified Bodies in the medical device sector. My clients often describe me as a collaborative team player with exceptional communication skills. Outside of work, I’m an avid mountain biker and hiker, embracing the outdoors with the same passion I bring to my professional life.

Robert F.
- Kansas

At the core, I’m a proud father of three, and much of my time is dedicated to them. In my spare moments, I’m passionate about college sports and enjoy unwinding with video games. I served 7 years on active duty in the Navy, which was followed by several years as a reservist. Prior to transitioning into my role as Sr. On-Site Program Coordinator, I spent about 15 years in customer service and sales, gaining valuable experience across various levels. In my current role, I am the primary point of contact for a wide range of inspector support services, including IT, payroll, time-off requests, and client data inquiries. At Spirit, my focus is on developing processes where none exist and refining procedures for larger-scale programs, ultimately creating a blueprint for sites to function independently in the future.

Robert F.
- China

Robert Feng joined SQA in 2012 and is one of our leading auditors in China. With 23 years of experience in quality assurance within the pharmaceutical and dietary supplement industries, Robert has conducted over 300 audits for clients in the healthcare sector. His expertise and dedication have made him an invaluable member of our team. Outside of work, Robert enjoys reading, traveling, and swimming. As a qualified and committed SQA Associate, we are proud to have him as part of our team!

Yigang S.
- New York

SQA has been the biggest part of my independent contractor business. Since partnering with SQA in August 2018, I have completed more than 80 supplier audits and were it not for COVID-19, I expect that number would be 100+. Working as an independent contractor has its rewards and challenges. “Flexibility” is a plus – I work twice as hard and take on as much work as possible, but I also spend more time with my family. I’m very proud of my family, and I’m very happy that, while working 60-80-hour weeks, I have lots of quality time with them. The other plus is that I love the work I do – while helping clients with projects and audits to meet their needs, I’m also continuously learning and making improvements. Thank you, SQA, for helping me make this all possible!

